The COVID-19 pandemic hit the world in early 2020 and the world hit back. With cash.
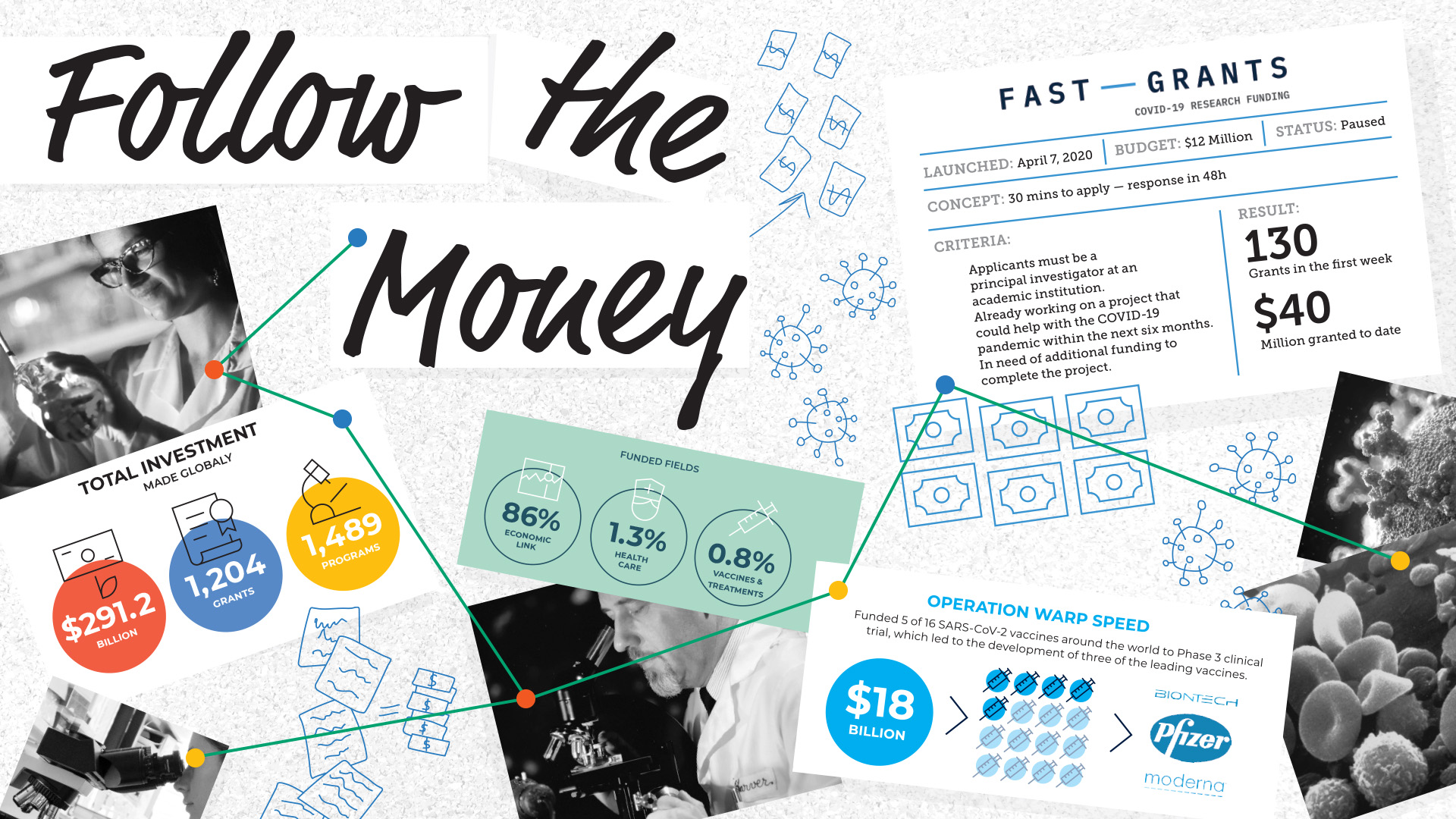
According to Devex, an independent media platform specializing in global development based in Washington, D.C., as of June 27, 2020, investments totaling U.S.$291.2 billion were made globally through 1,489 program announcements and 1,204 grants. This money didn’t go to health care research though: 86 percent of funding announcements had an economic link to their objectives, with just 1.3 percent clearly linked to health objectives and 0.8 percent for vaccines and treatments.
Yet health care research into the virus proceeded, and within nine months, two vaccines were ready to go. Research and development like that needs investment.
“Understanding who has been doing what is challenging, since there are no complete databases on global research and development. Some sources are proprietary, and in many cases, funding is non-transparent,” says Bhaven Sampat, an associate professor in the Department of Health Policy and Management at Columbia University. “However, the data we can piece together reveal a consistent picture of public-sector support for downstream research, development and production.”

Sampat’s research found the largest funding response came from the U.S. government, with roughly U.S.$15 billion of the U.S.$4 trillion allocated to the COVID-19 response given to R&D for vaccines and treatments. A worldwide rush of research on treatments and vaccines had begun, and as the world’s largest funder of biomedical research, the National Institutes of Health (NIH), took steps to coordinate the efforts of public and private
researchers globally, to focus on promising candidates and avoid duplicate research.
“Other global actors also contributed funding for therapies and vaccines,” Sampat says. “The European Union has provided financing from the European Investment Bank, member states such as France and Germany have provided research funding, and while data is unavailable from China and Russia, both appear to have made substantial investments in R&D and are responsible for seven of the fourteen vaccines in Phase 3 trials.”
PHILANTHROPY TO THE RESCUE
Philanthropic actors have also had a huge part in the response. Not only did philanthropies donate money to vaccine and treatment development but they also played a coordinating role, helping to facilitate the global scale-up. “In Latin America, the Carlos Slim Foundation supported at-risk manufacturing of the Oxford/AstraZeneca vaccine in Argentina and Mexico,” Sampat says.
At-risk contracts are advance market commitments: They are promises to purchase the product, enabling manufacturers to raise the financing to build the facilities and workforces necessary for emergency production. In vaccine manufacturing, there’s a regulatory aspect too. The Oxford/AstraZeneca vaccine was still awaiting approval, meaning the manufacturers would be turning out doses without knowing whether they’d pass regulatory scrutiny. Risky business.
RAPID RESPONSE?
“The COVID-19 pandemic tested the NIH’s ability to fund critical research to answer research questions that significantly affect public health and require urgent scientific clarity,” Tinglong Dai, professor of operations management and business analytics at John Hopkins University, says. Dai’s research focuses on the health care ecosystem and health care operations management. Dai looked into the funding awarded by the NIH for COVID-19 research, finding just 2 percent of the NIH-funded projects had anything to do with COVID-19 research in 2020.
“In 2020, COVID-19 research accounted for 5.3 percent of the annual NIH budget of $41.7 billion,” Dai says. “In the first three months of the global pandemic, a total of six grants were awarded for COVID-19 research, with the NIH spending a total of 0.1 percent of its annual budget on COVID-19 research in this time. By the end of 2020, that total had risen to 5.3 percent.”

Dai and his fellow researchers found that infrastructure and education accounted for 55.9 percent of NIH COVID-19 funding, yet many of the major clinical questions surrounding transmission were left unanswered, creating challenges for evidence-based policymaking. This may have influenced the public response to COVID-19 measures such as mask mandates and social distancing. The researchers also pointed out that the lack of rapid clinical research funding to understand COVID-19 transmission may have contributed to the politicization of the virus.
“Some of the most basic questions that were being asked of medical professionals in early 2020, such as how it spreads and whether masks protect individuals, went unanswered,” Dai says. “In the absence of evidence-based answers, political opinions filled that vacuum. As the largest research-funding arm of the federal government, the NIH has a responsibility to fund research that can address misinformation with evidence. A resilient health care system in times of crisis should be able to pivot funding toward specific grants answering critical gaps in knowledge.”

Dai says he believes the NIH should develop mechanisms to rapidly award funding to addressing scientific unknowns associated with sudden, large-scale health emergencies: “Supporting sound clinical research aimed at developing evidence-based recommendations is important for public policy and promotes public trust in the medical profession during a pandemic.”
Although the NIH response may have been sluggish, venture capitalists were quick off the mark.
READY, SET, GRANT!
Official advice from the NIH suggests that grant planning should begin nine months in advance of the deadline for the grant. That’s inefficient at the best of times, but needing to pre-empt a crisis no one saw coming makes it a disastrous way to award funding.
Fast Grants is an aptly named response to this funding issue, set up by Patrick Collinson, CEO of Stripe, and Tyler Cowen, economist at George Mason University. On April 7, 2020, Fast Grants launched a call for grant applications, offering a decision within 48 hours. It was immediately swamped, granting its entire budget of U.S.$12 million in less than a week.

The eligibility criteria were simple: Applicants must be a principal investigator at an academic institution, already working on a project that could help with the COVID-19 pandemic within the next six months, and in need of additional funding to complete the project.
In that first week, Fast Grants awarded more than 130 grants, offering the money “as fast as your university can receive it.” Fast Grants promised it would take less than 30 minutes to apply, and a decision would be made within two days, with Cowen making the final decisions to distribute the money. It was immediately obvious that there were thousands of projects just waiting for a final injection of cash, and the venture capitalists behind Fast Grants put up more money for a second call on July 12, 2020.
Applications are now paused “due to receipt of a very large number of qualified submissions,” according to the Fast Grants website. As of 2022, Fast Grants has awarded more than USD$40 million to Covid-19 research that can be finished within six months.
FAST RESULTS
Receiving the money may be super speedy compared with traditional funding avenues, but Fast Grants wanted super speedy results in return. “Most existing funding bodies focus on supporting longer-term work,” the Fast Grants website reads. “Given COVID-19’s human costs, speed is of paramount importance.”
Following successful applications, many projects were completed and contributed significantly to the global pandemic response: Wataru Akahata at Kyoto University and Erec Stebbins at the German Cancer Research Center worked on developing vaccines; Carolyn Bertozzi at Stanford University identified predictive biomarkers for COVID-19 disease progression; Steven Marc Friedman at University of Toronto investigated saliva as a test for SARS-CoV-2; and Alain Townsend at Oxford University looked into antibodies to the virus’ spike protein.
 IMAGE: Harry S. Truman Library and Museum
IMAGE: Harry S. Truman Library and MuseumThe National Defense Research Committee
Formed: June 27, 1940 Read more›››
Dissolved: June 28, 1941
Role: Coordinate, supervise and conduct scientific research.
Achievements: Provided $6.5 million to scientific research.‹‹‹ Read less
Collinson and Cowen were inspired by a similar funding mechanism dating back to the days of World War II.
The National Defense Research Committee (NDRC) was an organization created in 1940 to “coordinate, supervise, and conduct scientific research on the problems underlying the development, production, and use of mechanisms and devices of warfare” in the United States. In its 12 months of activity, it funded the research into some of the most important technology used during World War II, including radar and the atomic bomb. Although it became the Office of Scientific Research and Development in 1941 and was eventually terminated in 1947, in its one year of existence, the NDRC provided USD$6.5 million to scientific research. And it granted this money quickly.
“Within a week, the NDRC could review the project,” reads the memoir of Vannevar Bush, leader of the project in 1940. “The next day, the director could authorize, the business office could send out a letter of intent, and the actual work could start.”
Although radar and sonar received the bulk of the NDRC attention, arguably its most important project eventually became the Manhattan Project, the full-scale effort to produce nuclear weapons. No longer beholden to the US military for funds, the project ran full steam ahead with NDRC funds.
In a global conflict, time was of the essence. In a global pandemic, it’s the same story. Time mattered in the early days of the Second World War. Days mattered.
THE NEED FOR SPEED
Days mattered in the early days of the pandemic, too: Getting money to researchers was literally a matter of life or death.
Researchers at Columbia University suggest that had the U.S. begun social distancing just one week earlier, the country could have prevented 36,000 deaths through early May 2020.
“In major metropolitan areas, we found significant reductions in transmission when social distancing and other control measures were implemented,” Jeffrey Shaman, epidemiologist at Columbia University, writes in a paper published in 2020. “Our simulations indicate that had these same control measures been implemented just one to two weeks earlier, a substantial number of cases and deaths could have been avoided. Such dramatic reductions in morbidity and mortality due to more timely deployment of control measures highlights the critical need for an aggressive, early response to the COVID-19 pandemic.”
The U.S. response wasn’t entirely sedate, however. In May 2020, a program named Operation Warp Speed was initiated to accelerate the development, manufacturing and distribution of COVID-19 vaccines, therapeutics and diagnostics. The program promoted mass production of multiple vaccines and types of vaccine technologies, allowing for faster distribution if clinical trials confirmed one of the vaccines was safe and effective.
The plan anticipated that some of these vaccines would not prove safe or effective, making the program more costly than typical vaccine development, but potentially leading to the availability of a viable vaccine several months earlier than typical timelines.

By January 2021, Operation Warp Speed had put its U.S.$18 billion budget to good use, funding five of 16 SARS-CoV-2 vaccines around the world to Phase 3 clinical trial stage.The US threw money at the problem, and it paid off with the development of the Moderna and Pfizer/BioNTech vaccines.
It’s important to note, however, that the NIH was mandated by Congress to disburse its COVID-19 funds over a nearly five-year time frame. This allows for more planning and longer-term research, while negating the need for a huge change in funding processes.
With other initiatives such as Operation Warp Speed, it could be claimed that the NIH did not need to change the way it awards funding, especially when combined with a perhaps expected injection of billions of dollars in public-private collaborations and investment from the private sector.
WHEN PRIVATE AND PUBLIC PLAY TOGETHER
Although governments around the world struggled to respond to COVID-19, scientists and researchers made the most of expedited resources to tackle the virus.
In only a few weeks, Public-Private Partnerships (PPPs) were formed. These collaborative relationships mostly include governmental agencies and intragovernmental organizations (such as the World Health Organization as public actors, and university and research institutes, commercial pharmaceutical companies and professionals as private actors. Complementary expertise and knowledge is pooled, and the rewards are shared.
“We saw the rapid formation of PPPs to develop new vaccines for the novel coronavirus,” says Vijay Pereira, associate professor of humanities and social sciences at Khalifa University. “The private sector offered the funds and tapped into the knowledge base of partners from academic, public, and private-sector organizations to leverage each other’s strengths towards a common goal.”
In many ways, partnerships between academia and industry make sense: There are many societal problems in need of a technological solution, and industry is willing to pay for these solutions. Researchers with interests in line with public concerns can contribute to solving the problem, and industry can then get these solutions to the public.
To put it bluntly: Industry players spend money to make money and will spend that money on research that can ultimately make them more money. This is great for times of crises like the COVID-19 pandemic and the increasingly extreme climate change — crises that are a central risk to human prosperity and health.
But this leaves researchers looking into less industry-friendly science at a loss. Government funding is harder to get, and there’s no money to be made from the more esoteric scientific questions.
Industry funds applied-solutions-oriented science, not science for science’s sake (the so-called “blue sky” science).
The private sector offered the funds and tapped into the knowledge base of partners from academic, public, and private-sector organizations to leverage each other’s strengths towards a common goal.
– Vijay Pereira, associate professor of humanities and social sciences at Khalifa University
And yet there is value in science for its own sake, for research into questions that don’t have an immediate application. Value that goes beyond the potential much of this science will ultimately have (mRNA vaccine, anyone?). Converting this value from curiosity to cold hard cash is where the problem lies.
Unless you’re a billionaire research scientist, you’ll need external funding for your work. Traditionally, this involves you or your team bidding for funding, writing endless documents about your work and your ideas, with the application review process and administration taking months. If funded, projects are given a time frame: In three to five years, you should have an answer, a result.
Whether your proposal receives funding will rely heavily on whether your purpose and goals closely match the priorities of granting agencies.
The COVID-19 pandemic was the perfect example of science with immediate application being prioritized and a dramatic shift in the way research is funded.
RECOVERING FROM COVID-19
As money and focus were channeled into COVID-19 research over the past few years, it had to come from somewhere. In many cases, projects that would otherwise have been funded had to be overlooked in favor of grappling with an emerging crisis.
The National Institute for Health and Care Research (NIHR) in the United Kingdom funded research into COVID-19 with U.K. Research and Innovation (UKRI), a non-departmental public body sponsored by the Department for Business, Energy and Industrial Strategy; a public body, yes, but one intrinsically linked to the department overseeing business and industry in the United Kingdom. Industry-funded research, if you will.
At the same time, the NIHR is embedded in the U.K.’s National Health Service, meaning the research findings it funded were quickly provided to the NHS doctors and nurses to prevent and treat COVID-19.
“Under NIHR’s restart framework, urgent public-health research into COVID-19 continues to be our number one priority,” the NIHR website reads. “We do, however, recognize the importance of also investing in longer-term research.”
Part of this longer-term research is an investigation into the lessons learned during the pandemic on managing the recovery of research into other conditions.
“While the pandemic inevitably reduced the amount of research we have been able to do into other conditions, we have worked hard to maintain a diverse and active portfolio,” the NIHR website reads.
While research into other conditions continued, it was severely affected by a reduction in capacity and accompanying NHS services.
In December 2020, Cancer Research U.K. announced cuts of £45 million to its research budget. CRUK is responsible for roughly half of publicly funded research into cancer in the U.K. The Canadian Cancer Society predicted that the pandemic would cost them CA$100 million in lost donations during the ongoing financial year, which amounts to more than half their budget.

The American Cancer Society saw a decrease in revenue of around U.S.$200 million. The Association of Medical Research Charities predicts that it will take more than four years for spending in the sector to return to pre-pandemic levels, and a decade to rebuild lost capacity and capability.
While the NIH may have been slow to pivot toward funding COVID-19 related projects, it did not cut funding for cancer research, meaning NIH-funded research into other (equally critical) health care issues was able to continue during the pandemic. After all, it’s not like other diseases or health care problems disappeared with the arrival of COVID-19.
Unfortunately, much research into those problems was halted, not necessarily due to a lack of funding, but safety concerns. The vice-chancellor of Cambridge University instructed “unless it is related to COVID-19, all research undertaken on university premises will need to be paused.” Clinical trials around the world were also forced to stop as it was simply unsafe for patients to visit hospitals unless absolutely necessary, and medical staff were needed elsewhere to support care for COVID-19 patients. All this adds up to serious delays in finding treatments for other serious illnesses. How these projects will recover from the pandemic remains to be seen.
Short-term measures are bound to have a long-lasting impact on the research world. As funding decreased for non-COVID-19-related projects, recruitment had to stall, meaning there are quality researchers out there with no jobs. Lab closures mean projects can’t be completed by their deadlines, with grant money running out and PIs unable to pay their lab members. Not to mention the long-term economic downturn as a result of the pandemic means science funding could face longer-term impacts and cuts. The cancer charities seeing fewer donations is a direct result of a weakened economy.
Many of the world’s largest research funders did adapt their funding policies in response to the pandemic but these changes can’t have saved every project.
THE FUTURE OF FUNDING
The COVID-19 pandemic put a spotlight on health care research and its pathways for funding. It’s clear that research in academia would benefit from significant restructuring to deal with global challenges that require fast solutions.
“Our findings revealed critical flaws in the funding of critical public health research in the United States,” says Dai, the Johns Hopkins University professor who looked into COVID-19 funding. “Research responsiveness — that is, an infrastructure that allows for rapid and near-real-time learning in terms of clinical practice, public health measures and management principles — is a critical dimension of health care resilience.
“While, in the future, research infrastructure should be rapidly expandable and facilitated during times of crisis to better understand the impact and solutions, non-crisis-related research should not be halted, but supplemented by crisis-related research to minimize disruptions in other health care and public-health priorities. Additionally, surveillance of novel threats should be funded before an issue even arises, so we can be better prepared in the event of a new crisis.”
Research responsiveness — that is, an infrastructure that allows for rapid and near-real-time learning in terms of clinical practice, public health measures and management principles — is a critical dimension of health care resilience.
– Tinglong Dai
“The COVID-19 innovation system represents a departure from business as usual,” says Columbia’s Sampat. “Considering the remarkable progress to date, especially on vaccine development, this raises the question of whether this model is useful only for crisis times, or whether biomedical innovation policy in ‘normal’ times might productively incorporate some elements of the COVID-19 model as well.”
In the wake of the pandemic, we can all reflect on what we got right and what we got wrong. It’s clear that getting funding to scientists in a matter of days helped enormously in dealing with a global health threat, but the volume of applications for such grants proved how much science there is out there in need of just a final boost of investment.
A need for quick answers to some of the most pressing questions highlighted the burdensome process of applying for grants and accessing funding. The pandemic not only paused projects, but impacted researcher careers, especially those in the early stages, and now there may not be roles to return to.
“Applying the COVID-19 model beyond the pandemic would mean more public funding for late-stage research, clinical trials, development and manufacturing,” Sampat says. “The pandemic has demonstrated that effective biomedical research policy does not end with drug and vaccine development alone.”